Common Unrecognised Factors Affecting Dark Adaption
By Dr Surjit Singh Wadhwa
Recently much has appeared in the amateur astronomy literature on the effects of intrusive white light on dark adaptation. Some correspondents have also raised the question of the effects of smoking and alcohol, two very common "abuses" among amateur astronomers. Intrigued by some of the letters and reports I decided to do some definitive library-based research. The results were much more surprising and interesting than I had imagined and feel they may be useful for the amateur community at large.
The report is divided into four parts - firstly, a brief overview of dark adaptation and the physiology of the eye. The second and third parts deal with specific effects of alcohol and smoking, the final part presents a summary and conclusions.
The eye possesses a remarkable capability to become sensitive to light. Light perception of the eye can increase by a factor of 10 billion from full sunlight to the least light perceptible. There are two major components of dark adaptation namely dilatation of the pupil and the photochemical alterations of the retina.
The pupil can alter in size in response to light by constricting and to the dark by dilating. The upper and lower limits of pupil size are 3mm and 7mm. This variation is equal to over 500% alteration in light entering the pupil from fully constricted to fully dilated. Although not adequate to achieve full dark and light adaptation, pupil size alters rapidly and gives, within 15 to 20 seconds, an appreciable increase in the ability to see in dim light. One major factor which must be understood however is that the pupil size controls the amount of light actually available for the retina to "see" with and an inability to fully dilate the pupil will severely limit light perception even with a fully adapted retina.
The mechanism of dark adaptation involves an interplay between Rhodopsin, Retinene and Vitamin A, all of which are located in the rods of the retina. Rhodopsin (also known as visual purple) is a photosensitive protein which accumulates (synthesised) in the rods during exposure to darkness. Rhodopsin is converted to Retinene on exposure to the dimmest of light. During this interaction with light an electrical impulse is generated which the brain "sees" as light. Retinene is an unstable molecule and will convert back to Rhodopsin if the light exposure was of short duration (< 5 minutes) and there is sufficient Oxygen and glucose available. This conversion back to Rhodopsin is rapid and does not appreciably reduce dark adaptation. If however the exposure to light is of long duration (>7 minutes), even dim light, then the Retinene is converted to Vitamin A. Vitamin A is a stable molecule and the regeneration of Rhodopsin from Vitamin A is slow and is accomplished over a number of minutes. Thus prolonged exposure to light (>7 minutes) is required to significantly alter dark adaptation (Campbell et al. 1955).
Given the above physiology of dark adaptation it is clear why initial dark adaptation is reported to occur over 20 minutes. Over this time the accumulated vitamin A is converted to Retinene and then to Rhodopsin. The physiology also explains the lack of loss of dark adaptation reported by some amateurs following short exposures even to bright light. In these cases the Rhodopsin although bleached by the light exposure is quickly regenerated from Retinene which did not go on to be converted to the stable vitamin A.
Clearly, from the physiology and physics, any mechanism which hinders the function of nerves, muscles and the protein synthesis will have a detrimental effect on achieving and maintaining dark adaptation. As is evident from the discussion above, both protein synthesis and neural responses are important in vision and dark adaptation. Neural response is important in initiating and maintaining a dilated pupil and for conducting the retinal image to the visual cortex. Protein synthesis is important in amplifying the visual signal presented to the retina. Protein synthesis as a physiological process is dependent on adequate oxygenation (which in turn is dependent on blood flow and the ability of the blood to carry and off load oxygen) and also on the presence of various co-factors such as glucose. Below is a review of the effects of alcohol and smoking on some of the parameters which influence dark adaptation as well as on the process of dark adaptation as a whole.
Effects of Alcohol
In a study looking at pupillary dilation in subjects who use alcohol regularly to those who either do not use alcohol or only sparingly, Rubin et al (1977) found that both the rate of increase in size of the dilating pupil and the amplitude are higher in those who do not regularly abuse alcohol compared to those who regularly consume alcohol. The study found that the maximum pupillary diameter of non-users was 6.2mm while those using alcohol regularly the maximum diameter was 5.7mm. This small variation may not seem significant, however if one looks at light gathering capacity, the non-users gather nearly 20% more light than regular alcohol users. The effects are illustrated in figure 1. The above study did not look at the acute effects of alcohol but rather the effects of chronic alcohol use. To look at the acute effects, the same authors (Rubin et al. 1980) conducted a study to observe the pupillary response to dark and stress (exposure to cold - common stressor for amateur astronomers). In response to stress the pupil dilated more when the subjects were not under the influence of alcohol than when the same stressor was applied after the subjects had consumed 60mL of spirits (two standard drinks).
 Figure 1.
Figure 1.
Alcohol reduces both the speed and extent to which the pupil dilates. The maximum size of the pupil in regular alcohol users was found to be 5.7mm while in non-users it was 6.2mm.
Alcohol appears to have little effect on retinal blood flow and oxygen carrying capacity of the blood. Being a neuro-depressant it does, however, have an effect on the transmission of visual information to the cerebral cortex. Zrenner et al (1986) demonstrated that alcohol decreased the light peak (strength of the nerve impulse to the cortex) by values between 3% and 79%. These effects were seen at blood alcohol levels of 0.07% and 0.16% (3 - 6 standard drinks). The result of the reduced light peak was an increase in the error of both light and colour detection with the Farnsworth - Munsell 100 Hue test error score rising from 26 to 79. The effect of the alcohol on the eye was most marked 30 - 95 minutes after ingestion however there was still an average 14% reduction in light peak at 130 minutes after ingestion. Other investigators like Watten (1996) and Toffolon (1990) have demonstrated significant reduction in contrast sensitivity, stereoacuity, visual field loss and binocular vision after ingestion of alcohol to achieve breath alcohol level of only 0.05 - 0.1% (3 - 6 standard drinks).
It is clear from the above that the neuro-depressive effects of alcohol do affect the visual function. The major effects appear to be on the pupil which dilates slower and to a much lesser extent after exposure to alcohol. This effect alone could result in 20% reduction in light gathering capacity. The effects on perception are difficult to gauge but given the loss of strength of the nerve impulses to the cortex and the resulting increase in visual perception errors at low blood alcohol levels, one can safely make the assumption that alcohol will reduce the ability to "see" all there is to "see".
Effects of Smoking
The effects of smoking and associated exposure to carbon monoxide are even more profound than those relating to alcohol described above. Inhaled cigarette smoke causes tissue hypoxia (reduced oxygen to a body organ). The toxicity is caused by disturbing the oxygen carrying capacity of the blood as well as the oxygen delivery to the tissues. This is because the inhaled carbon monoxide binds to haemoglobin (oxygen carrying protein) thus preventing oxygen from binding and it also prevents any oxygen that is already bound form being released into the tissues. Additionally there is evidence that inhaled carbon monoxide binds to intercellular ligands and shuts down protein synthesis (Stewart, 1976; Goldbaum et al, 1975).
The eye is part of the central nervous system and is highly sensitive to the lack of oxygen (McFarland, 1970). Since oxygen consumption of the eye is increased in the dark (Riva et al, 1983), special attention has been devoted to investigating the effects of carbon monoxide on visual function.
Before looking at the effects of smoking on dark adaptation as a whole, I will briefly discuss the effects of smoking on some of the finer aspects of the visual physiology.
Morgado et al (1994) examined the effects of smoking on retinal blood flow and the ability of the retina to alter blood flow in response changes in the level of blood oxygen. They found that smoking caused a significant decrease in retinal blood flow, blood flow was found to have decreased by almost 10% after smoking 2 cigarettes. The ability of the retina to respond to changes in blood oxygen is termed its oxygen reactivity, the higher the reactivity the better the retina can adjust blood supply. The study found that oxygen reactivity decreased from 28% before smoking to 19% after smoking. In a small subgroup of diabetics, smoking resulted in complete loss of ability of the retina to adjust its blood supply.
The retina has numerous pigments (proteins) which respond to light. Hammond et al (1996) examined the effects of smoking on mean retinal pigment density. They did not look at the acute effects of smoking but rather the long-term effects by studying a group of smokers and a group of non-smokers. The results showed that the average pigment density for the non-smokers was 0.34, but in smokers the mean pigment density was markedly reduced at 0.16. The study also found that the reduction in pigment density was dose related i.e. the more the subject smoked the greater the loss in pigment density.
Unlike alcohol, which appears to affect mainly the neurological aspects of vision, smoking is more inclined to affect the ability of the eye to see by reducing the oxygen supply and inhibiting protein synthesis. A number of studies have been done to directly examine the effect of smoking on dark adaptation. von Restorff et al (1988) performed an elegant study looking at the effects of smoking (by way of carbon monoxide exposure) on the velocity of dark adaptation and on the light sensitivity of the dark-adapted eye. The study was done in smokers and non-smokers so that effects of chronic smoking could also be studied.

Figure 2.
Smoking regularly and acutely increases the time required for the eye to dark adapt.
The time between the end of a blinding flash and the detection of a light stimulus of 1mlux was taken as a measure of the velocity of dark adaptation. Under control conditions (i.e., no smoking), subjects who were regular smokers needed 5.9 minutes to dark adapt while non-smokers required only 4.9 minutes. The results imply that smokers needed 20% more time than non-smokers to dark adapt. Both groups were then tested under conditions of high carbon monoxide (smoking). The blood level of Carbon Monoxide achieved was similar to that achieved after smoking 2-4 cigarettes. In both groups the time to dark adaptation increased, in the non-smoker’s time to adapt increased from 4.9 minutes to 5.7 minutes while in the smoking group adaptation time increased from 5.9 minutes to 6.9 minutes. The results clearly demonstrate that habit smoking reduces the ability of the eye to dark adapt quickly, it also shows that acutely, smoking can increase dark adaptation time by 16% in both smokers and non-smokers. These results are presented in figure 2
To assess the light sensitivity of the dark-adapted eye, the threshold of perception of a light stimulus was investigated. Under control conditions, the dark adapted non-smokers were able to detect a light source of 46 microlux, while the dark adapted smokers could not detect light below 69 microlux, an almost 50% increase above non-smoker thresholds. Under conditions of carbon monoxide exposure (smoking) there was no significant increase in threshold of non-smokers however in smokers the average threshold increased to 100 microlux. A good video camera has a sensitivity of 0.1 lux (100 microlux), the human eye in a smoker is thus only as sensitive as a good video camera. The results are illustrated in figure 3. Similar results were found by Calissendorff (1977) who found that smoking increased visual threshold at 10 and 15 minutes into dark adaptation.
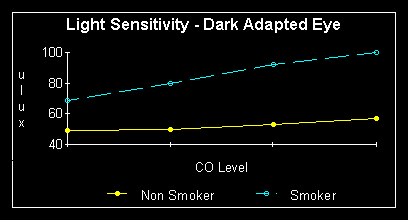
Figure 3.
Smoking, especially regular smoking, decreases the ability of the dark adapted eye to detect light.
Summary and Conclusion
In conclusion, dark adaption is slow initially but is only lost after prolonged exposure to light, short exposure to even bright light will not mean a long recovery time for dark adaption. Alcohol reduces the ability to see faint objects by decreasing the light entering the eye by up to 20% and then reducing the ability of the brain to fully appreciate contrast. Smoking on the other hand reduces the ability of the eye to see by mechanisms unknown but most likely by inhibiting the ability of the retina to achieve full amplification. I think it is clear from the above that as amateurs we should avoid alcohol and smoking to maximise our potential to observe detail and, secondly, we should not be too concerned about the occasional stray white light (even bright light), it will not ruin our well-earned dark adaptation
References
- Calissendorff B. Acta Opthal., 55: 261-268, 1977
- Cambell P. and Rushton J. J Physiol., 130: 131-147, 1955
- Goldbaum LR., Ramirez RG., et al. Aviat.Space.Environ.Med., 46:1289-1291, 1975.
- Hammond BR Jr. Vision Res., 36(18): 3003-3009, 1996
- McFarland RA. Ann N.Y. Acad Sci., 174: 301-312, 1970
- Morgado PB., Chen HC., et al. Ophthalmology., 101(7): 1220-1226, 1994
- von Restorff W. and Hebisch S., Aviat. Space. Environ. Med., 59: 928-931, 1988
- Riva CE., Grunwald JE., et al. Invest. Ophthal. Vis. Sci., 24: 737-740, 1983
- Rubin LS., Gottheil A., et al. J Stud Alcohol., 38(11): 2036-2048, 1977
- Rubin LS., Gottheil A., et al. J. Stud Alcohol., 41(7): 611-622, 1980
- Stewart RD. J. Occup. Med., 18: 304-309, 1976
- Toffolon G. J. Stud. Alcohol., 51(2): 108-113, 1990
- Watten RG. Ophthalmic. Physiol. Opt., 16: 460-466, 1996
- Zrenner E. Doc. Ophthalmology., 63(4): 305-12, 1986

